Understanding Matrix Effects and Ion Suppression in Liquid Chromatography Mass Spectrometry
Matrix Effect & Ion Suppression in Liquid Chromatography Mass Spectrometry
Matrix Effects are the suppressing or enhancing properties of a co-eluting compound from a liquid matrix on the primary signal response of the target analyte. In several liquid chromatography-tandem matrix mass spectrometry (LC-MS) biological studies, Matrix Effects can suppress the ion intensity by interfering with target analyte ionization. Compounds with high mass, polarity, and basicity are typical candidates to trigger matrix effects.
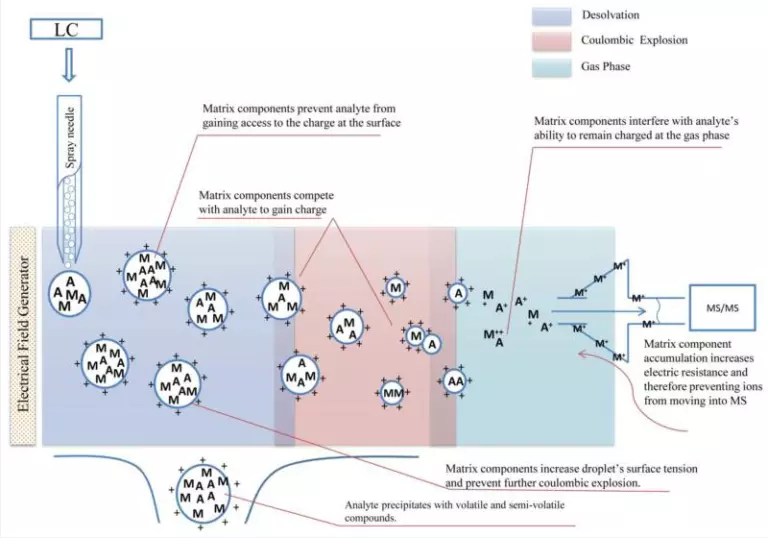
Matrix components can deprotonate and neutralize the analyte ions produced in the liquid matrix, causing ion suppression. Matrix Effects may also be caused by co-precipitation of the analytes with less-volatile and heavy compounds. Under these circumstances, the efficiency of droplet formation in liquid matrix gets affected. High viscosity interfering compounds in a biological matrix could increase the surface tension of the charged droplets and further prevent evaporation. Additionally, matrix compounds can reduce the stability of the analyte ions produced in the gas phase. Also, the accumulation of charged matrix components in front of a quadrupole mass analyzer entrance could lead to charging issues, thus preventing the analyte ions from moving into the mass analyzer.
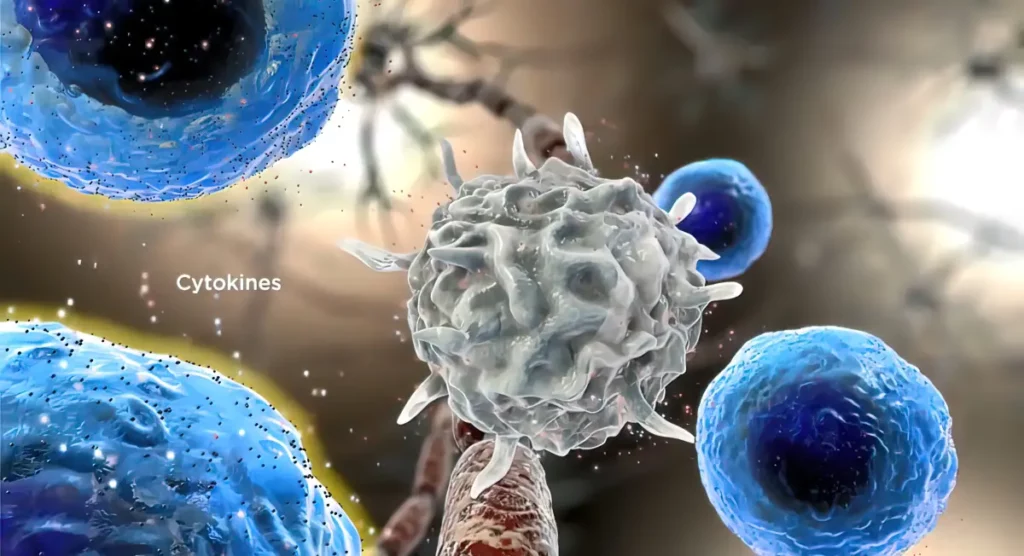
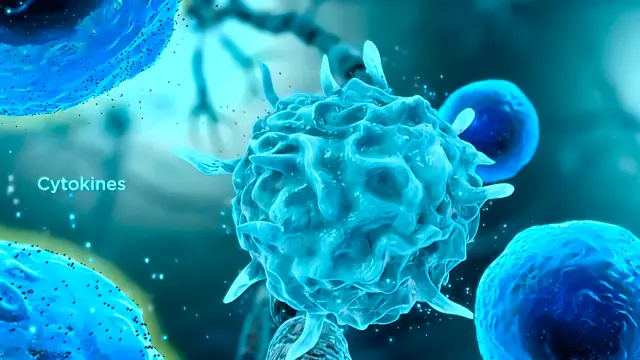
Mechanisms of matrix effects in Electrospray Ionization (ESI)
In LC-MS studies, matrix interference occurs when co-eluting compounds from a biological matrix mass spectrometry influence the primary signal response of the target analyte. This Matrix interference can severely impact analytical accuracy in ion suppression mass spectrometry.
The phenomenon of ion suppression can be attributed to matrix components that deprotonate and neutralize analyte ions in the liquid phase in LC-MS analyses, a critical issue in ion suppression mass spectrometry.
The liquid matrix components, particularly those with high viscosity that increase surface tension can potentially affect the efficiency of droplet formation in liquid chromatography.
For understanding and mitigating the impact of matrix effects on analyte quantification, ion suppression mass spectrometry techniques are essential. These techniques are pivotal in addressing and correcting Matrix interference.
Researchers always aim to minimize matrix mass spectrometry effects through cleaner sample preparation methods and by carefully adjusting chromatographic conditions to optimize the separation and minimize interference.
Figure 1 below summarizes the proposed mechanisms of matrix effects in Electrospray Ionization (ESI).