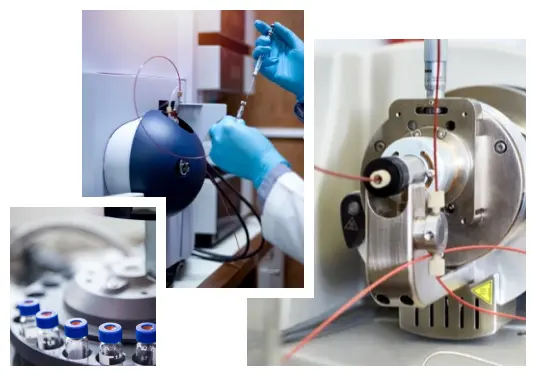
LC-MS Method Setup and Sample Analysis during Drug Development
The LC-MS assay method is used during various stages of the drug development cycle. Most notably, it is used in the following phases:
Drug Discovery:
Over the past couple of decades, high-performance liquid chromatography combined with tandem mass spectrometry (LC-MS/MS) analysis has emerged as one the preferred analytical technique for new drug discovery assays.
Preclinical Toxicology:
Studies range from early lead optimization to dose range finding studies followed by Tox studies. These studies involve primarily plasma and tissue samples of rodent and non-rodent species.
Typically, GLP toxicology studies are performed using fully validated LC-MS methods. Theoretically, this could be accomplished using HPLC with UV and other detection methods like fluorescence, but these detection methods aren’t as selective and require extensive sample preparation. Use of MS detection in multiple reaction monitoring mode (MRM) is quite selective, and therefore drugs can be analyzed in biological matrices with simple cleanup steps. Also, LC-MS assay can be developed relatively faster for a new drug and are generally very robust and reliable.
Clinical trials:
These studies range from Phase 1 (dose escalation studies like single ascending and multiple ascending dose, healthy volunteer studies) to Phase III, Phase IV and post-marketing studies. Matrices involved in the analysis range from plasma, serum, blood, urine, feces, and tissues from different organs.
Over the last 10-15 years, the LC-MS/MS method has rapidly become the method of choice during clinical trials. At the early stages of clinical development, it is essential to analyze plasma samples from clinical trials for understanding ADME properties of the drug and ensuring data consistency with preclinical PK studies. Thus, LC-MS/MS methods are developed and validated for the drug analysis and testing in human biological fluids.
Liquid Chromatography:
Here’s how the entire LC-MS assay works:
As the first step, the liquid chromatography is used to separate the proteins, nucleic acids, or other endogenous material in complex biological matrices. There are only minor differences between HPLC (High-Performance Liquid Chromatography) setup with other detectors compared to how HPLC-MS is set up. Among other aspects, these differences include the column length and flow rate. Benefiting from the mass spectrometer, chromatography columns used in LC-MS/MS tests are much shorter than those used in standard HPLC. For example, the regular columns are about 100-300 mm long while those in the LC-MS method are only about 30-50 mm long. Consequentially, the flow rate for LC-MS is slower than the flow rate in the HPLC technique which is 1ml/min. Since the LC-MS combines the advantages of HPLC with mass spectrometry, it can be used for a wider variety of drug analysis as well as environmental testing and food analysis.
Mass Spectrometry:
The instrument consists of three major components:
- Ion Source: Produces gaseous ions from the substance being analyzed.
- Analyzer: Resolves the ions into their mass components according to their mass-to-charge ratio.
- Detector System: Detects the ions and record relative abundance of each of the resolved ionic species.
First step in the mass spectrometric analysis is the production of gas phase ions of the compound by electron ionization. The molecules that come out of the chromatography column are highly pressurized. Since the mass spec units operate in a vacuum, the continuous flow cannot be detected by the spectrometer. Therefore, the liquid eluted from the chromatography column must be passed through an interface before it can be transferred to the mass spectrometer. The most common interfaces are atmospheric pressure photo-ionization (APPI) systems, atmospheric pressure chemical ionization (APCI) systems, and electrospray ionization (ESI) systems. The liquid that passes through the interface gets nebulized into a spray, after which it is ionized and transferred to the mass spectrometer.
The mass spectrometer measures the mass-to-charge ratio of the ions and then records the relative abundance of each ion type, producing a mass spectrum of the molecule. The equipment displays results in the form of a plot of ion abundance versus mass-to-charge ratio.