Meso Scale Discovery is a robust platform to measure molecules in complex biological samples. Meso Scale Discovery assays can help you profile biomolecules such as intracellular signaling proteins and cytokines. Meso Scale Discovery combines electrochemiluminescence detection with multi-array technology to provide unsurpassed sensitivity and multiplexing capacities, making it an ideal detection platform for drug discovery and development studies. The Meso Scale Discovery multiplex system offers a wide range of assay options, including MSD immunogenicity assay, MSD cytokine assays, MSD PK assay, MSD ADA assay, and MSD cell based assay.
With 20+ years of experience developing sensitive immunoassays, NorthEast BioLab is committed to providing robust Meso Scale Discovery multiplex assays for measuring analytes such as biomarkers, phosphoproteins, and cytokines in complex biological matrices. Moreover, we offer MSD assay development and MSD assay optimization services to support and accelerate your drug development timelines. As your assay partner, we help you develop robust MSD assay protocols for high-quality MSD analysis even in the most challenging situations.
Our MSD multiplex assays provide greater analytical performance and customer value. We design numerous MSD assays, such as MSD biomarker assay, MSD ADA assay, MSD cytokine analysis assay, MSD cell based assay, and MSD PK assay, based on a variety of pharmacological and biomedical needs. Additionally, our MSD multiplex ELISA assays eliminate technical issues associated with other platforms while offering superior reliability, higher accuracy and sensitivity, and faster workflows. Besides, Multi-Spot panels in MSD ELISA assays provide multiplexing capacities to analyze multiple analytes simultaneously.
A majority of Meso Scale ELISA assays are sandwich-based assays. MSD labs employ assays such as Meso Scale PK and Meso Scale cytokine multiplex assays to evaluate drug concentrations in biological samples. In MSD cytokine analysis, the MSD method can be processed conveniently through 96-well or 384-well microtiter plates. Let us now dive deep into the working of MSD assays such as Meso Scale pharmacokinetics assay and Meso Scale PK assay.
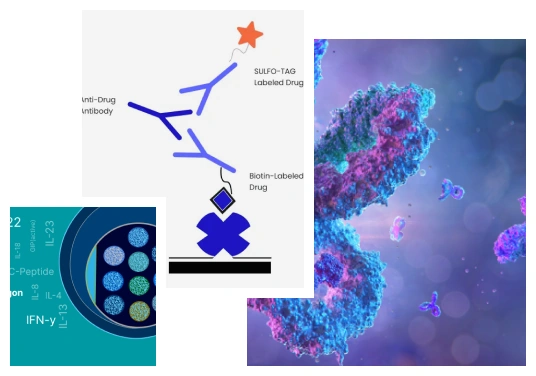
MSD has a proprietary platform for measuring analytes in complex study samples. This technology has assays such as MSD cytokine multiplex assays to profile cytokines and cell signaling molecules, having a direct impact on drug development initiatives. MSD cytokine analysis consists of two crucial components, electrochemiluminescence detection, and Multi-array technology.
Electrochemiluminescence detection provides enhanced sensitivity, a broader dynamic range, and convenient sample analysis. It employs electrochemiluminescent labels that generate light when stimulated under appropriate conditions. This reaction is the basis of all MSD assays to measure important biomedical molecules. Moreover, MSD assays are highly reliable and provide accurate data through MSD data analysis in a broad range of sample types.
Multi-array technology is the combination of electrochemiluminescence and multiple arrays. This union brings speed and highly dense biological information. When applied to multi-spot plates, this technology can precisely quantitate several analytes in a single assay volume. Multi-spot MSD plates have arrays within each well. Each well can have up to 10 spots. Hence, a 96-well or 384-well assay plate can analyze numerous study samples, saving precious biological samples and reagents.
Moreover, MSD has two reliable instruments in its arsenal, MESO SECTOR S 600MM and MESO QuickPlex SQ 120MM. Both these instruments offer robust performance and enhanced reliability. Besides, these instruments work on efficient software designed for analyzing high-quality study data.