Why use ELISAs to measure mAb concentrations in pharmacokinetic studies?
Enzyme-Linked Immunosorbent Assays (ELISAs) can be useful for measuring monoclonal antibody concentrations in pharmacokinetic studies. They are versatile immunoassays widely used in pharmacokinetic studies to precisely measure mAb concentrations.
- They offer high sensitivity, specificity, and a wide dynamic range, making them suitable for both low and high antibody levels.
- They are known for their accuracy and reproducibility, adaptable to high-throughput screening,
- They are versatile enough to measure various antibody classes and isotypes.
However, successful use depends on understanding the idiosyncrasies of the specific antibodies and tailoring and optimizing the assay format and reagents to work with the antibody.
Scientists at NorthEast BioLab have the expertise in assay development and Good Laboratory Practice (GLP) standards to develop customized GLP-compliant assays for pharmacokinetic studies of mAbs, ensuring precise measurement of antibody concentrations in biological samples.
A project involving measurement of mAb concentrations in clinical or preclinical samples, at NorthEast Biolabs will go through three phases:
- Assay Development and Optimization
- Assay Validation
- Sample Analysis
Assay Development and Optimization
During assay development, scientists at NorthEast design an assay in the matrix required and, when possible, at the sensitivity requested. Development will involve determining:
- the appropriate type of ELISA (Direct, Indirect, Sandwich, Competitive)
- the potential range of the assay
- the appropriate capture and detection antibodies, their optimal concentrations, and the appropriate dilution schemes for achieving those concentrations
- the optimal incubation times and temperatures
- the appropriate assay block
- the optimal assay diluent(s) and establishing minimum required dilution (MRD)
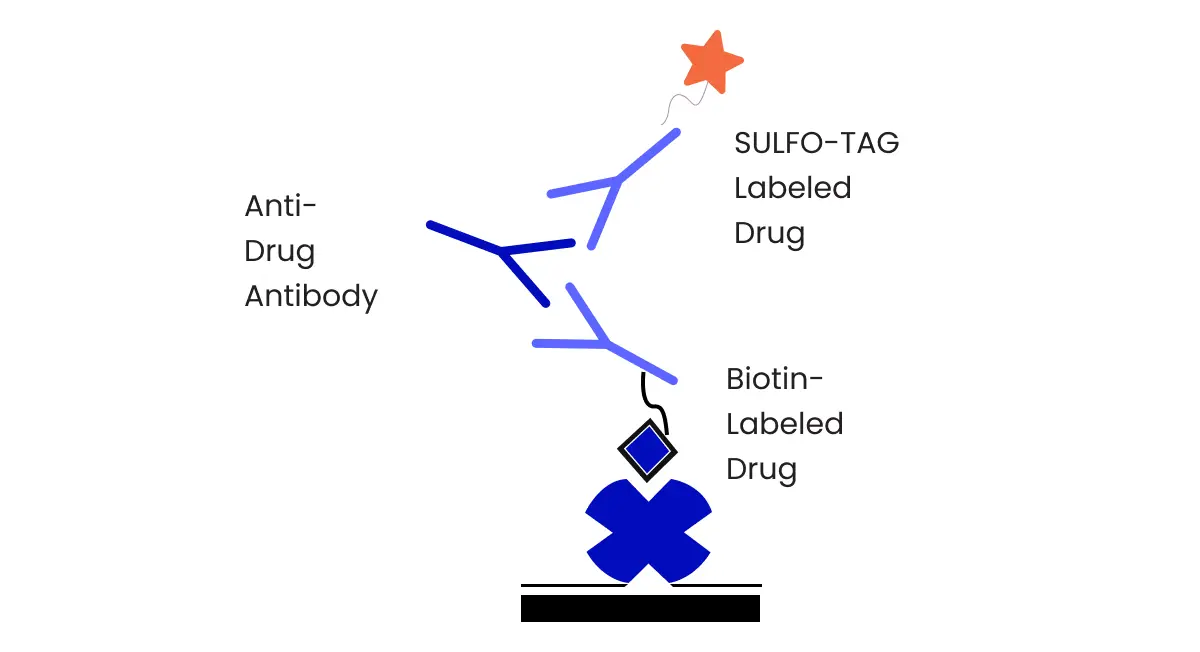
1) Determining the Appropriate Type of ELISA
Choosing between Direct, Indirect, Sandwich, or Competitive ELISAs depends on the assay’s objectives and the nature of the analyte being measured.
- Direct ELISA is used when a specific antibody is available for direct binding to the target analyte, making it a straightforward choice for assays where such antibodies are accessible and when high specificity is required.
- Indirect ELISA is valuable when the target analyte lacks specific antibodies but can bind to a general capture antibody. It amplifies the signal through the use of a secondary antibody, enhancing sensitivity.
- Sandwich ELISA is ideal for analytes with multiple binding sites or when specific antibodies for capture and detection are available. It offers high sensitivity and specificity by sandwiching the analyte between the two antibodies.
- Competitive ELISA is employed when detecting the presence of an analyte that can compete with a labeled analog. This method is suitable for measuring small molecules, antigens, or haptens.
The choice between these ELISA types hinges on factors like the analyte’s characteristics, available antibodies, desired sensitivity, and specificity requirements.
Developing assays with specific sensitivity levels can be challenging due to the idiosyncrasies of the target molecule, antibody affinity, and sample matrix effects.
Many of these decisions will be made with support from assay batches using the checkerboard technique to systematically explore a range of reagent concentrations, ensuring that you select the combination that maximizes assay performance.
2) Determining the potential range of the assay
Determining the potential range of an assay involves assessing the anticipated concentration levels of the analyte of interest and ensuring that the assay can effectively capture data across this range.
3) Determining the appropriate capture and detection antibodies and their appropriate concentrations
Selecting the appropriate capture and detection antibodies for an immunoassay involves careful consideration of their specificity and affinity for the target analyte. It often necessitates screening multiple antibodies to identify the most suitable pair. Optimal concentrations for these antibodies are determined through checkerboard experiments where varying combinations of antibody concentrations are tested to find the point of maximal signal while minimizing background noise. The appropriate dilution schemes are subsequently devised to achieve these optimal concentrations, ensuring that the assay attains high sensitivity and specificity for accurate analyte detection.
4) Determining the best incubation times and temperatures
Selecting the optimal incubation times for an immunoassay involves conducting an initial range of experiments to find the right balance. Consideration of antibody-antigen interaction kinetics helps identify times that maximize specific binding while minimizing non-specific background noise. Fine-tuning within a narrowed range and subsequent validation with representative samples ensures the assay’s reliability and precision in accurate analyte detection.
5) Determining the best blocking agent
Selecting the appropriate blocking agent for an immunoassay involves careful considerations to optimize the assay’s specificity and reduce non-specific binding. First, it’s essential to evaluate the characteristics of the assay and the nature of the samples. Factors such as sample matrix composition and potential interfering substances should guide the choice of a blocking agent. Common blocking agents include bovine serum albumin (BSA), non-fat dry milk, or proprietary blockers. Additionally, one must assess whether the assay requires a single blocking step or a multi-step blocking strategy. Comprehensive testing and optimization of the chosen blocking agent, including concentrations and incubation times, are essential to ensure it effectively minimizes non-specific interactions while preserving the signal generated by specific antibody-antigen binding. Ultimately, the goal is to create an environment that maximizes assay specificity and minimizes background noise, ensuring accurate and reliable results.
6) Choosing the right assay diluent and establishing minimum required dilution (MRD)
Selecting the appropriate assay diluent for an immunoassay is a critical consideration to maintain the assay’s sensitivity and specificity. The diluent should be compatible with the assay components and maintain the stability of antibodies and antigens. Factors such as pH, ionic strength, and the presence of potential interfering substances in the sample matrix must be taken into account. In some cases, specialized assay diluents may be needed to address specific challenges, such as reducing matrix effects or enhancing signal-to-noise ratios. Rigorous testing and optimization of the chosen diluent, including its formulation and concentration, and establishing MRD are essential to ensure it supports the assay’s overall performance and reliably delivers accurate results. Ultimately, the goal is to create an environment that maximizes antibody-antigen interactions while minimizing interference, thereby ensuring the assay’s precision and effectiveness.
Standard NorthEast Biolab’s Indirect ELISA Protocol
Coat Plate (2 Hrs. / Overnight)
Add Calibrators, Controls, Samples
Add Substrate
Stop Reaction
Measure Absorbance
ELISA Method Validation
Once the assay range has been determined, the reagents and diluents have been chosen, optimized, and their concentrations determined, the method is ready for validation. A assay validation will demonstrate that the specific method is accurate, reliable, and suitable for its intended purpose. It involves assessing parameters like accuracy and precision, specificity, sensitivity, dilution linearity, assay range and, stability through assays designed to measure those parameters based on the protocol developed during assay development and optimization.
- Accuracy and Precision: Accuracy is assessed by comparing measured values to known reference values or standards. Precision is determined by testing repeatability (intra-assay) and intermediate precision (inter-assay) to ensure consistent results.
- Specificity: Specificity is evaluated to confirm that the method accurately measures the target analyte without interference from other substances. It may involve analyzing potential cross-reactivity or matrix effects.
- Sensitivity: Sensitivity is determined by measuring the limit of detection (LOD) and limit of quantitation (LOQ), which represent the lowest concentrations of the analyte that can be reliably detected and quantified.
- Dilution Linearity: Dilution linearity assesses whether the method maintains a linear relationship between concentration and response when samples are diluted to various levels.
- Assay Range: The assay range is defined by testing the method’s performance across a range of analyte concentrations to ensure accurate measurements within that defined range.
- Stability: Stability assesses the method’s ability to remain reliable over time and under varying conditions, including controlled variations in experimental parameters or sample storage conditions.
The results are documented in a validation report, ensuring the method’s quality and compliance with regulatory standards when necessary.
Pharmacokinetic Sample Analysis
Once the method has been developed, it can be used to analyze analyte concentrations in the pharmacokinetic samples. Optical readings, generated by antibody-antigen complex formation, are converted into analyte concentrations using calibration curves. Quality control measures ensure accuracy and precision, and the results are securely stored electronically for reporting and further analysis, supporting the calculation of pharmacokinetic parameters and informed decision-making in drug development and clinical research.
Pharmacokinetic Data Analysis
The obtained data, including sample concentrations, are analyzed statistically. This analysis allows for the calculation of essential pharmacokinetic parameters, including but not limited to Cmax (maximum concentration), Tmax (time to maximum concentration), AUC (area under the curve), and t1/2 (elimination half-life). These parameters offer crucial insights into drug absorption, distribution, metabolism, and elimination, guiding dose optimization, dosing regimens, and therapeutic decisions. Advanced modeling techniques and statistical tools are often applied to extrapolate data, assess inter-individual variability, and predict drug behavior under different conditions. Ultimately, pharmacokinetic data analysis informs critical decisions in drug development, ensuring the safety and efficacy of pharmaceutical compounds in clinical practice.
The results of the pharmacokinetic analysis are documented in a report, which includes the calculated pharmacokinetic parameters for each subject or participant.