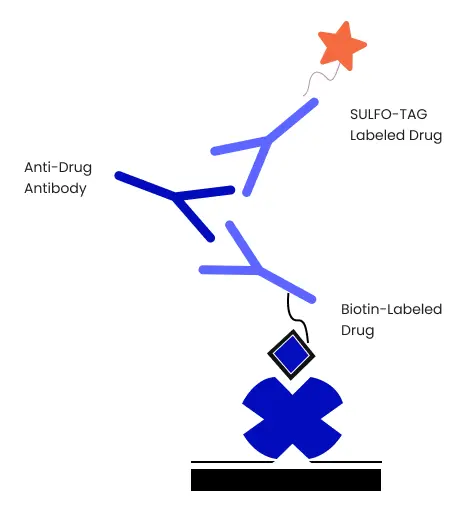
Preliminary Assay Experiments To Optimize Bridging Immunogenicity Assay
This section discusses the preliminary experiments to optimize ADA bridging assays. ADA bridging ECL assay also requires optimizing the biotinylated and Sulfo-Tag labeled drug and the minimum required sample dilution. The four primary optimizing assay experiments are:
- Optimization of testing sensitivity and reagent concentration
- Testing matrix tolerance
- Testing free drug tolerance
- Employing acid dissociation
Let us first begin with optimizing testing sensitivity and reagent concentration.
1. Optimization Of Reagent Concentrations And Testing Sensitivity
This experiment consists of two types; testing sensitivity using a Master Mix and checkerboard optimization.
A. Testing sensitivity using a Master Mix containing equal concentrations of biotinylated and SULFO-TAG labeled drugs.
This experiment tests three or more concentrations of Sulfo-Tag and biotinylated labeled drugs with an ADA titration series.
Reagent Preparation
Anti-Drug Antibody Samples
Prepare 25% and 100% normal or naive plasma or serum. Dilute the plasma or serum in an assay diluent and prepare ADA dilution series in 25% and 100% normal or naive plasma or serum. The recommended concentrations of ADA test assays are 10000, 2500, 625, 156.3, 39.1, 9.8, 2.4, and 0 ng/ml. Each assay well consists of 25 µL of the study sample. If needed, one may adjust the test concentrations accordingly.
SULFO-TAG and Biotinylated Drug Mastermix
Prepare 3-4 different concentrations of Sulfo-Tag and Biotinylated drug Master Mix with equal concentrations of both in assay diluent. Each assay well consists of 50 µL of Master Mix. The recommended amounts of labeled drugs to test antibody therapeutic drugs are as follows:
- For Streptavidin Gold plates, the concentrations are 1.0, 0.5, 0.25, and 0.125 µg/ml of Sulfo-Tag labeled and biotinylated antibody
- For High Bind Avidin Gold plates, the concentrations are 2.0, 1.0, 0.5, and 0.25 µg/ml of Sulfo-Tag labeled and biotinylated antibody
Notes/Precautions
- Avoid exceeding the recommended amount of drug specified for each plate type
- For High Binding Avidin Gold plates, do not cross 0.6 pmoles of drug per well. While with Streptavidin Gold plates, do not cross 0.3 pM of drug per well
- For any therapeutic other than an antibody, the concentration of the Sulfo-Tag and the biotinylated drug should be adjusted according to the molecular weight of the drug
An Exmple of MSD Protocol
- Add 25 µL of standard or sample and 50 µL of Master Mix in each well
- Seal the plates and incubate overnight at 4˚C or 1-2 hours at RT
- Add 150 µL of blocking buffer in each well during the incubation step
- Post incubation, remove the blocking buffer and wash it with a washing buffer
- Transfer 50 µL to another plate and incubate for 1 hour at RT with shaking at 300-700 rpm.
- Wash the wells
- Add 150 µL of 2X Read Buffer T to each well and read the plates
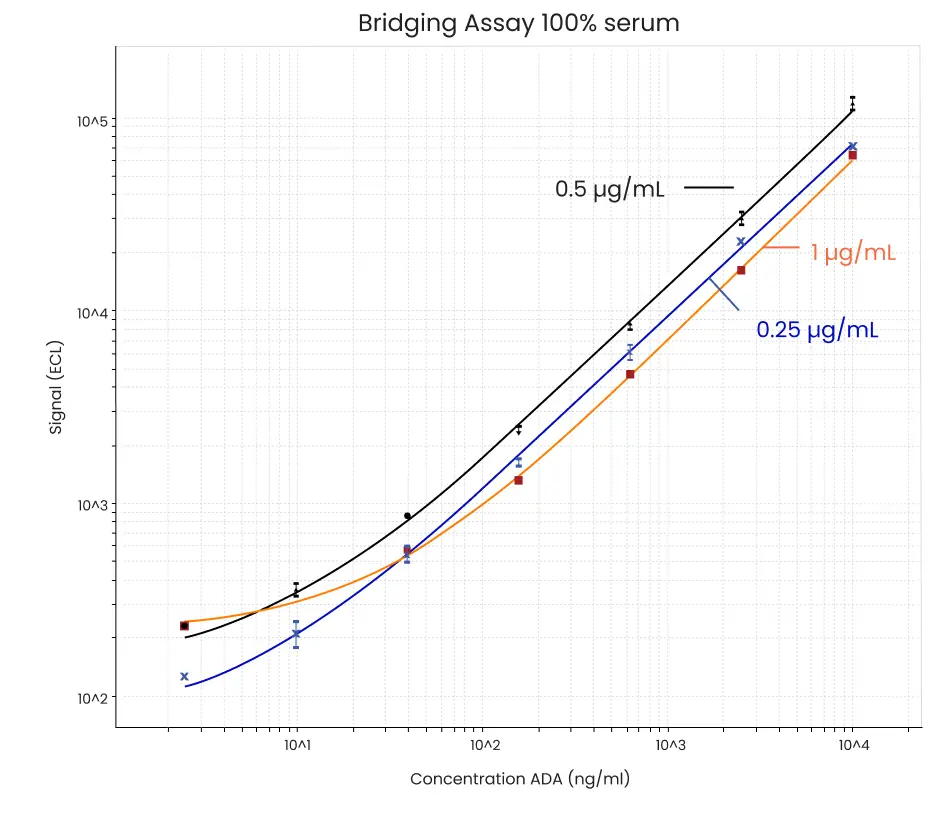
Figure 2. Example data from sensitivity experiment using equal amounts of biotinylated and SULFO-TAG labeled drug using a Streptavidin Gold plate. In this example, further studies were performed using a master mix where the final amount of biotinylated and SULFO-TAG labeled drug added per well of the plate was 0.5 µg/mL each. LLOD of the assay under these conditions was 3 ng/mL.
B. Checkerboard optimization of concentrations of biotin-labeled and SULFO-TAG labeled drugs.
Often relative affinities of the Sulfo-Tag labeled drug and biotinylated drug for ADAs may differ. Here, the 1:1 ratio of Sulfo-Tag labeled and biotinylated drug Master Mix may not be optimal. Hence, the current experiment follows a checkerboard titration to test different concentrations of Sulfo-Tag labeled and biotinylated drugs. These concentrations in checkerboard assays may range from zero to mid-range
ADAs.
Reagent Preparation
Anti-Drug Antibody Samples
Mimic the sample by preparing 500 ng/ml ADA standard in naive or normal serum. Prepare control by adding no ADA in the naive or normal serum. Each well contains 25 µL samples. Initial studies can be performed with 50% serum. The following table is a sample plate layout for assessing sensitivity using checkerboard titration.
| Biotinylated drug |
2μg/mL |
1μg/mL |
0.5μg/mL |
0.25μg/mL |
0.125μg/mL |
0μg/mL |
2μg/mL |
1μg/mL |
0.5μg/mL |
0.25μg/mL |
0.125μg/mL |
0μg/mL |
|
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
| Sulfo-Tag Drug |
4μg/mL |
A |
500 ng/mL Anti-Drug Antibody in serum |
0 ng/mL Anti-Drug Antibody in serum |
| 2μg/mL |
B |
| 1μg/mL |
C |
| 0.5μg/mL |
D |
| 0.25μg/mL |
E |
| 0.125μg/mL |
F |
| 0.062μg/mL |
G |
| 0μg/mL |
H |
Biotinylated Drug
Use assay diluent to prepare six different biotinylated drug concentrations. Each well will consist of 25µL. The following biotinylated drug concentrations are recommended for antibody therapeutics.
- For Streptavidin Gold plates: 2, 1, 0.5, 0.25, 0.125, 0 µg/mL
- For High Bind Avidin Gold plates: 4, 2, 1, 0.5, 0.25, 0 µg/mL
SULFO-TAG Labeled Drug
For Sulfo-Tag labeled drug, use assay diluent to prepare eight different Sulfo-Tag labeled drug concentrations. Again, each well will consist of 25 µL. The following Sulfo-Tag labeled drug concentrations are recommended for antibody therapeutics.
- For Streptavidin Gold plates: 4, 2, 1, 0.5, 0.25, 0.125, 0.62, 0 µg/mL
- For High Bind Avidin Gold plates: 8, 4, 2, 1, 0.5, 0.25, 0.125, 0 µg/mL
Notes/ Precautions
- Follow the recommended amount of drug per well and do not exceed that amount for individual plate type
- For biotinylated drugs using Streptavidin Gold plates, do not add more than 0.3 pmoles per well
- For biotinylated drugs using High Bind Avidin Gold plates, do not add more than 0.6 pmoles per well
An Example of MSD Protocol
- Add 25 µL of the Sulfo-Tag labeled drug and 25 µL of the biotinylated drug to each well
- Add 25 µL of the standard to each well (Standard = 0.g.mL or 500 ng/mL ADA in serum)
- Seal the plates. Incubate at RT for 1-2 hours with moderate shaking
- During incubation, add 150 µL of blocking solution
- Remove the blocking solution and transfer 50 µL to the streptavidin Gold plate from each well of the polypropylene plate
- Seal and incubate at RT for 1-2 hours with shaking
- Wash the plate three times with the wash buffer
- Add 150 µL of 2X Read Buffer T in each well and read the plate.
2. Immunogenicity Testing Services Matrix Tolerance
The matrix tolerance can now be assessed after determining optimal concentrations of Sulfo-Tag labeled and biotinylated drugs in the first set of experiments.
Reagent Preparation
Mastermix
Use the assay diluent to prepare 6 mL of the optimized Master Mix. Each well receives 50 µL of Master Mix. Notably, if a checkerboard titration is used to optimize the biotinylated and Sulfo-Tag labeled drug, the optimal concentration should be halved while preparing the Master Mix solution.
Matrix Dilution
Prepare the dilution series in assay diluent. Immunogenicity examples of the sample matrix dilution series is 100%, 50%, 25%, 12.5%, 6.25%, and 3.125% plasma or serum ADA concentration prepared in assay diluent.
Samples
Prepare a dilution series of ADA in each plasma or serum concentration. The
recommended ADA concentrations are 10000, 2500, 625, 156.3, 39.1, 9.8, 2.4, and 0 ng/mL. Moreover, it is also possible to select a suitable concentration range based on the data from previous sensitivity evaluations. Each well will consist of 25 µL of ADA samples.
An Example MSD Assay Protocol
- Mix 50 µL of Master Mix with 25 µL of standard or sample and add to each well of a polypropylene plate. Seal and incubate at RT for 1-2 hours with shaking or at 4˚C overnight
- During incubation, add 150 µL of blocking solution into the wells of the Streptavidin Gold plate. Seal and incubate at RT for 30 minutes with shaking
- Remove the blocking solution and wash the Streptavidin Gold plate. Transfer 50 µL of incubation mixture onto the Streptavidin Gold plate from the earlier polypropylene plate. Seal and incubate at RT for 1 hour with shaking
- Wash the plate with wash buffer
- Add 150 µL of 2X Read Buffer T and read the plate
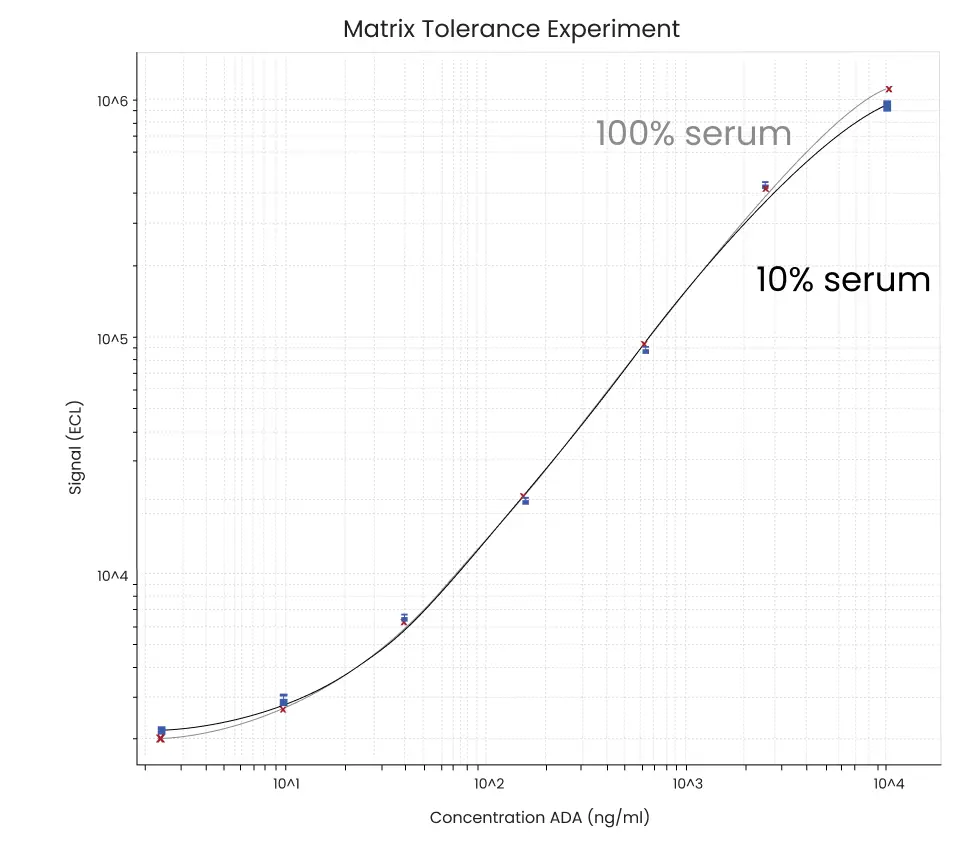
Figure 3. Example data from matrix tolerance experiment. In this example, there is little difference between the assay carried out in 100% and 10% serum.
3. Testing Free Drug Tolerance
Drug interferences can arise when high-affinity immune complexes form between the ADA and the drug in the sample. However, MSD immunoassay bridging assays are more robust against drug interferences. The antibody bridging assay principle uses a homogeneous solution phase incubation that significantly reduces the effects of drug interferences.
Free or circulating drugs can bind to ADAs and hinder the detection of anti-drug antibodies. However, the longer incubation format of MSD immunoassay bridging assays facilitates the dissociation of unlabeled drug and ADA complexes. This dissociation allows the Sulfo-Tag labeled and biotinylated drug to attach with the ADA in the sample.
One may enhance the tolerance against free drugs by employing a larger sample dilution. However,larger sample dilution may result in higher interaction of the ADAs with the labeled drug. MSD immunoassay bridging assay can tolerate pre-treatment methods such as acid/base neutralization. The following section discusses the immunogenicity testing services of free drug tolerance and will help how you would monitor the progress of a neutralization reaction.
Reagent Preparation
Mastermix
Use assay diluent to prepare 6 mL of Master Mix solution. The Master Mix solution will contain optimized concentrations of Sulfo-Tag labeled and biotinylated drugs. Each well will consist of a 50 µL Master Mix.
Anti-Drug Antibody Samples
Prepare dilution series for ADAs. The final concentration in serum will be 2X. The recommended ADA concentrations are 20000, 5000, 1250, 312.5, 78.1, 19.5, 4.8, and 0 ng/mL. Each well will consist of 25 µL of the sample.
Drug
Similarly, the dilution series of unlabeled drugs will have a final concentration of 2X. Recommended unlabeled drug concentrations for antibody drugs are 100, 25, 6.25, 1.56, 0.39, and 0 µg/mL. On the other hand, the recommended unlabeled drug concentrations for other immunogenicity of therapeutic protein are 600, 150, 37.5, 9.3, 2.3, and 0 nM. The required volume of each solution is 800 µL. Moreover, based on earlier PK analysis or expected concentrations of circulating drugs, one may test other concentrations of the unlabeled drug.
An Example MSD Protocol
- Add 50 µL of 2X free drug and 50 µL of 2X anti-drug antibody in a polypropylene plate. Seal and incubate at RT for 1 hour with moderate shaking
- Add 50 µL of Master Mix and 25 µL of ADA and unlabeled drug in another polypropylene plate. Seal and incubate at 4˚C overnight with moderate shaking
- Add 150 µL of blocking solution to the Streptavidin Gold plate. Seal and incubate at RT for 30 minutes
- Remove the blocking solution and wash the Streptavidin Gold plate with a wash buffer
- Transfer the incubation mixture onto the Streptavidin Gold plate. Seal and incubate at RT for 1 hour with shaking
- Wash the plate three times with the wash buffer
- Add 150 µL of 2X Read Buffer T and read the plate
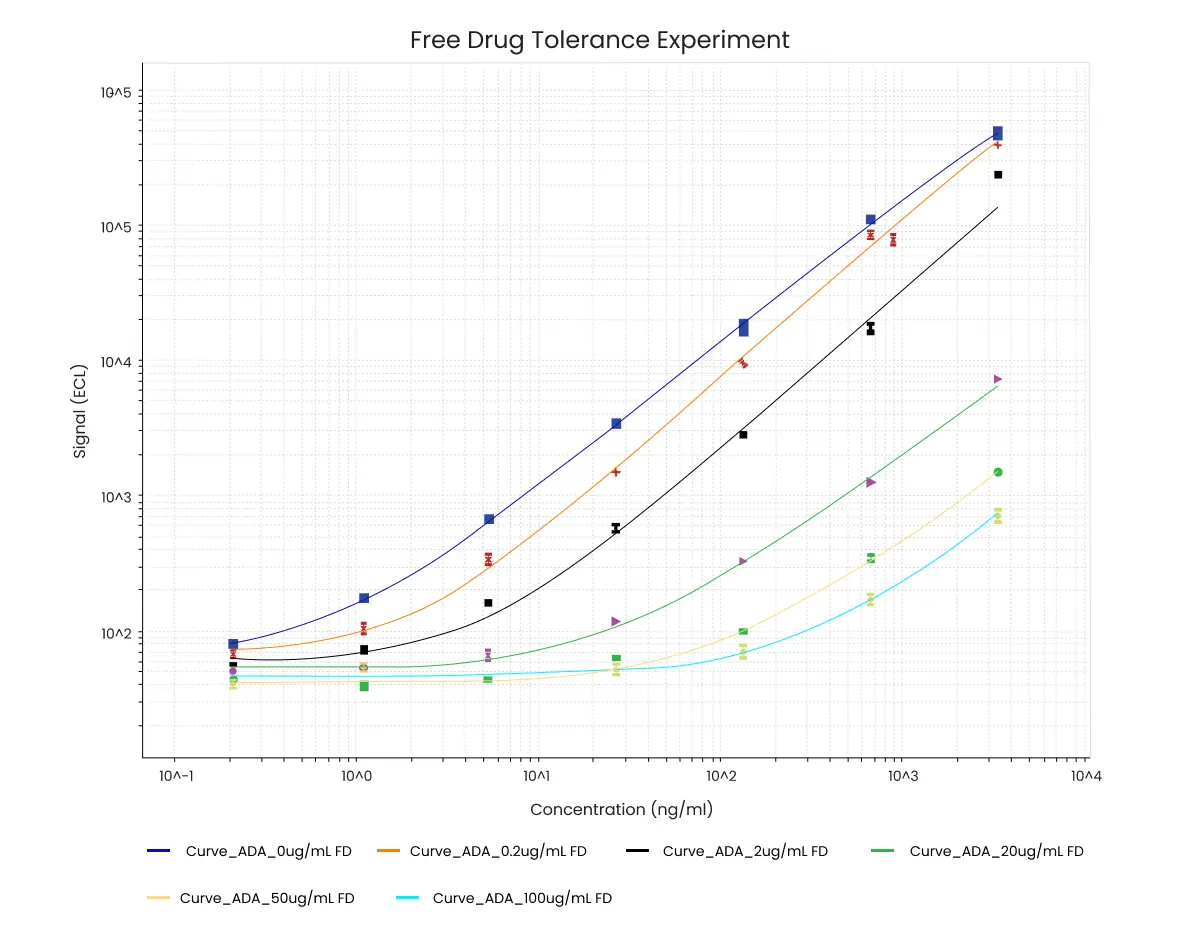
Figure 4. Example data from free drug tolerance experiment. The concentration of ADA (ng/mL) is indicated on the x-axis. In this example 150 ng/mL ADA is still detectable in the presence of 100 µg/mL unlabeled drug.
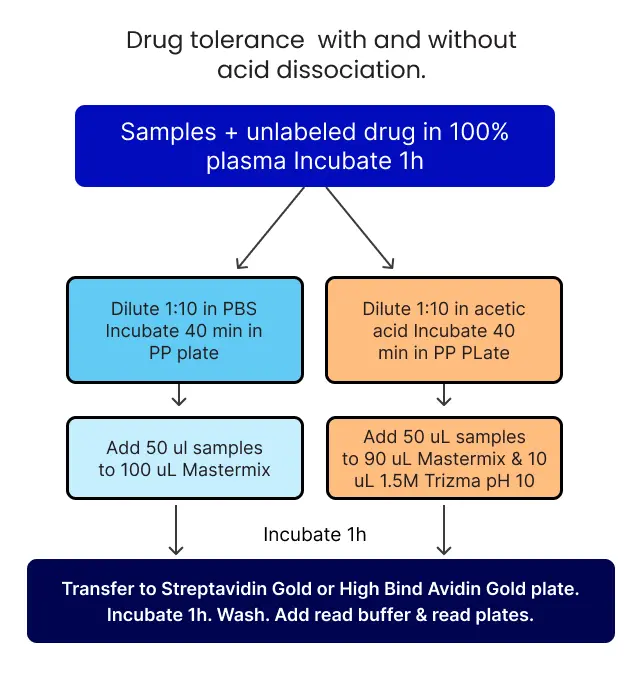
4. Acid Dissociation To Improve Drug Tolerance
Acid dissociation is a robust technique for dissociating high-affinity immune complexes to improve drug tolerance of ADA assays. In this technique, an acid treatment dissociates the ADA complex with the drug, and then it is neutralized using an excess of biotinylated and Sulfo-Tag labeled drugs.
Additional Reagent Required
- 1.5M Trizma Base with pH 10
- 300 mM Acetic Acid
Reagent Preparation
Mastermix
Use assay diluent to prepare 12 mL of Master Mix. The master mix will contain optimized concentrations of Sulfo-Tag labeled and biotinylated drugs. Each assay well will consist of a 90 µL Master Mix.
Anti-Drug Antibody Samples
Prepare ADA dilution series with a final serum concentration of 2X. Recommended 2X ADA concentrations are 20000, 5000, 1250, 312.5, 78.1, 19.5, 4.8, and 0 ng/mL. Each assay well will consist of 25 µL ADA samples.
Drug
Let the final serum concentration of the unlabeled drug dilution series be 2X. Recommended unlabeled drug concentrations for antibody drugs are 100, 25, 6.25, 1.56, 0.39, and 0 µg/mL. And for other therapeutics, the recommended unlabeled drug concentrations are 600, 150, 37.5, 9.3, 2.3, and 0 nM. Moreover, one may consider additional unlabeled drug concentrations based on PK results or expected concentrations of circulating drugs.
An Example MSD Protocol
- Add 50 µL of 2X free drug and 50 µL of ADA in a polypropylene plate. Incubate at RT for 1 hour with moderate shaking
- Take a new polypropylene plate and add 180 µL of 300 mM acetic acid or PBS in each well.
- Add 150 µL of blocking solution to a Streptavidin Gold plate. Seal and incubate at RT for 30 minutes
- Add 90 µL of Master Mix to another polypropylene plate. Add 12 µL of neutralizing solution for acid treatment or PBS for control well. Transfer 50 µL of the study sample to the Master mix well. Incubate at RT for 1-2 hours with shaking
- Remove the blocking solution and wash the Streptavidin Gold plate with a wash buffer. Transfer 50 µL of sample from the polypropylene plate onto this Streptavidin Gold plate. Seal and incubate at RT for 1 hour with shaking
- Wash the plate three times with a wash buffer
- Add 150 µL of 2X Read buffer T and read the plate
Notes
- During MSD assay protocol development, ensure that the pH of neutralized samples is neutral following neutralization and acid dissociation. One may adjust the volume of the neutralizing solution accordingly.
- Sometimes acid dissociation treatment may suppress positive control signals. It may also revoke the monoclonal antibody control responses
Figure 5. Overview of drug tolerance experiment with and without acid dissociation.
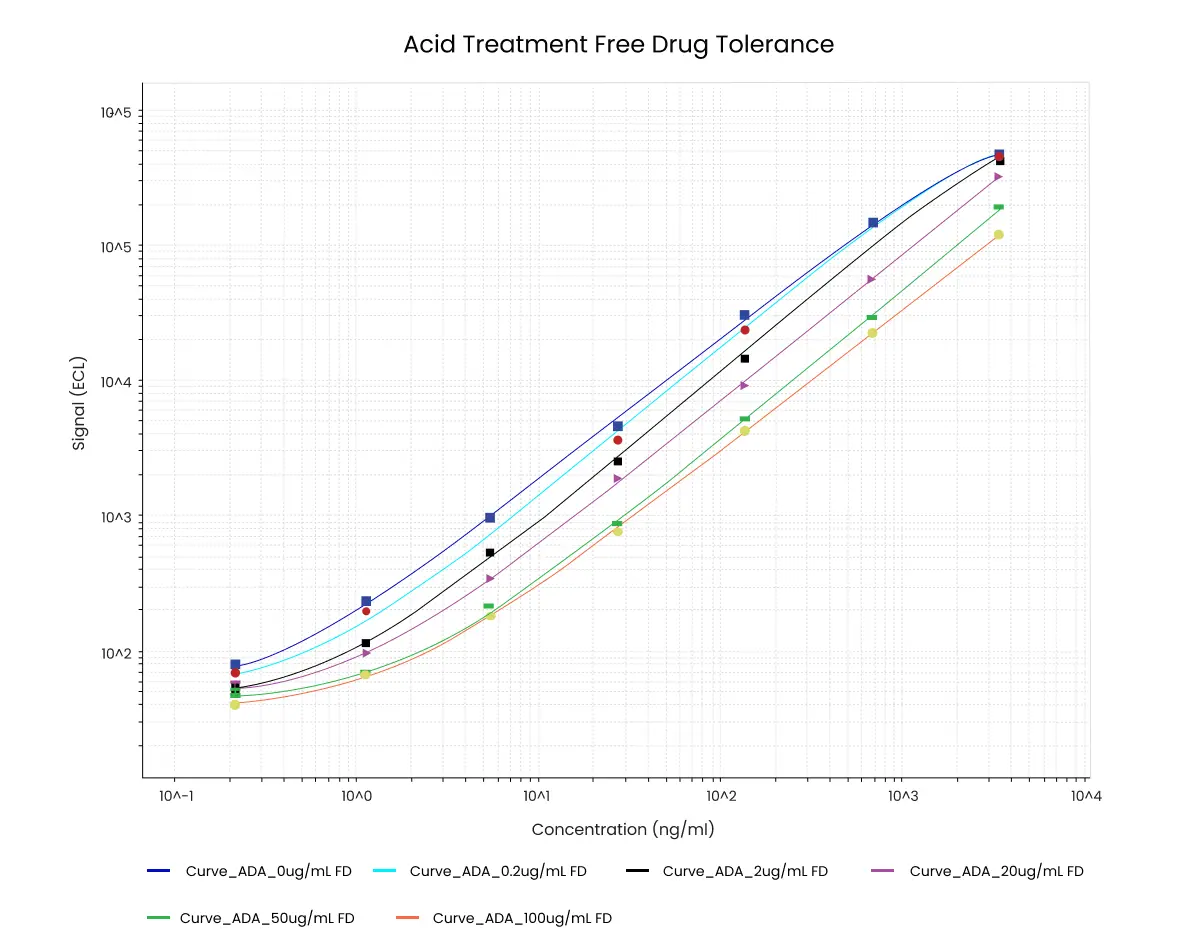
Figure 6. Example data from free drug tolerance experiment with acid dissociation. The concentration of ADA (ng/mL) is indicated on the x-axis. In this example 1 ng/mL ADA is still detectable in the presence of 100 µg/mL unlabeled drug.
Discussion and Troubleshooting
One may improve the performance of MSD immunogenicity assays in several ways. The drug tolerance of bridging MSD immunoassays can be improved by extending the incubation time of the Sulfo-Tag labeled drug, biotinylated drug, and sample overnight. Besides, larger sample dilutions or increased levels of Sulfo-Tag labeled and biotinylated drugs can be used to reduce drug interferences.Immunogenicity testing in clinical trials benefits significantly from these optimizations. Providers of immunogenicity bioanalytical services often implement these strategies to enhance assay sensitivity and specificity. Let us now understand some more recommendations for enhancing ADA assay performance, often utilized in immunogenicity testing services.
Streptavidin Gold and High Bind Avidin Gold Plates
- Streptavidin Gold plates are ideal for assay development. Post-assay development, one may test other plate types.
- For Streptavidin Gold plates, ensure the final biotinylated reagent is less than 0.3 pmole per well
- For High Bind Avidin Gold plates, ensure the final biotinylated reagent is less than 0.6 pmole per well
Signal levels
- The expected background signals for Sector PR instruments are 200-300 counts, whereas the count is 60-100 for Sector Imagers
- The maximum signal should be less than 1 million counts. This level is necessary as the dynamic range of all Sector instruments max outs at 1 – 1.5 million counts.
Shaking During Incubation
Shaking of plates is recommended during the incubation period. Shaking the assay plates increases the diffusion kinetics and helps reach the binding equilibrium in a shorter time. However, the shaking speed must be consistent to reduce assay variability.
Read Buffer concentrations
Use the Read Buffer T at 2X concentration. This concentration decreases the background signal and thus reduces the variability observed in the absolute signal.
Troubleshooting
High Backgrounds
Non-specific Sulfo-Tag labeled drug binding may elevate background signals. One may identify non-specific binding by running the assay without Sulfo-Tag labeled drug. Besides, control experiments with Sulfo-Tag labeled drug in assay diluent and sample matrix should be conducted to verify whether non-specific binding is due to matrix-induced effect or linked to the drug. Moreover, other blocking solutions and assay diluents must be tested to prevent non-specific binding.
Interaction between Sulfo-Tag labeled drug and biotinylated drug in the absence of plasma or serum may give rise to a higher background signal. Drug aggregates may generate non-specific interactions. The aggregation status of Sulfo-Tag labeled and biotinylated drugs can be assessed using various techniques such as dynamic light scattering and non-denaturing gel electrophoresis. Sometimes, this event may be
caused by a particular lot or batch of pharmaceutical drugs.
Storage conditions are crucial. They may lead to drug aggregation. Hence, labeled drugs must be stored at the designated temperature and buffer to maintain their stability.
Low Assay Signals
Low assay signals can arise from inefficient labeling or the inability to form a bridge between the ADA and Sulfo-Tag labeled and biotinylated drug.
Poor labeling is often due to the presence of interfering substances such as glycine, tris, and histidine. Hence, ensure the drug is in a carrier-free and amine-free buffer before labeling.
Limited reactive primary amine groups can also lead to a low or poor association of Sulfo-Tag and biotin. These limitations can arise from several factors, including limited protein sequence, linkage to polysialic acid, and blocked because of extensive modifications. In these situations, one may raise the pH of the labeling reaction to incorporate higher labeling ratios.
Overlabeling may result in drug precipitation. Precipitation happens likely with highly positively charged and lower molecular weight proteins due to charge neutralization. If the drug precipitation is evident, one may use lower labeling ratios. Addressing these issues is crucial for accurate immunogenicity testing in clinical trials.
Incomplete biotin removal also generates poor assay performance. This poor result is because free biotin competes with the biotinylated drug for binding. Hence, remove all unincorporated biotin after the biotinylation reaction. Ensuring thorough biotin removal is essential for reliable immunogenicity testing services and accurate results in immunogenicity testing in clinical trials.
Assay variability and signal reproducibility
Various factors influence inter and intra-plate reproducibility. These factors include:
- Read buffer concentrations
- Inconsistent shaking speed
- Dissociation rates
- Loss of drug activity
- Not following recommended reagent concentrations
- Inconsistent pipetting
- Variability during plate washing
- Plate lots
Exceeding recommended volumes may generate higher signal variability. Hence, as discussed earlier, the final biotinylated drug should not exceed 0.3 pmole per well for the Streptavidin Gold plate and 0.6 pmole per well for the High Bind Avidin Gold plate.
Higher Read Buffer concentrations give higher signals. The recommended concentration is 2X MSD Read Buffer T. Besides deionized water should be used to dilute the Read Buffer. Differences in the evaporation of stock buffers or preparation of Read Buffer may lead to differences in assay results. Moreover, Read Buffer must be used and stored at RT.
Often differences among operators or pipetting variability give inconsistent results. Calibrate all pipettes, and assess repeater pipettes for each dispense.
Different shaking speeds may affect assay signals by influencing diffusion rates and binding kinetics.
Hence, plate shaking speed must be consistent for optimal signal reproducibility.
Contaminated or blocked pins may generate inconsistent results with automated plate washers. Keep the plate washers clean and maintain them regularly. Often flipping the orientation of the plate solves most problems related to plate washers.
In an in vitro immunogenicity assay, Sulfo-Tag labels that are close to the bottom of the well generate assay signals. Generally, assay components are at equilibrium before the final wash step. However, if the plate is left in the Read Buffer or wash buffer for an extended period, the assay components may form a new binding equilibrium. This dissociation and reassociation decrease the assay signal. Hence, MSD assay plates should not be left in any buffer for more than the predetermined times.
Immunogenicity testing in clinical trials is crucial for assessing potential immune responses to biotherapeutics. Utilizing reliable immunogenicity bioanalytical services ensures accurate and reproducible results. Effective immunogenicity testing services help maintain the integrity of clinical trial data. For successful immunogenicity testing in clinical trials, it is imperative to avoid prolonged exposure to buffers. High-quality immunogenicity bioanalytical services and experienced immunogenicity testing services are essential for minimizing variability and achieving consistent assay performance.
Background decreases in the presence of unlabeled drug
On the other hand, assay background decreases in the presence of unlabeled drugs. This decrease is because the unlabeled drug competes for binding with the Sulfo-Tag labeled drug or non-specifically binds to the Sulfo-Tag labeled drug itself. Hence, control experiments must be conducted to determine the binding of unlabeled drugs.
False positives
Matrix-induced bridging of Sulfo-Tag labeled and biotinylated drugs by heterophilic antibodies, rheumatoid factor, soluble receptors, or target proteins may induce false positive results. Conduct control experiments using a closely related molecule for rheumatoid factor or heterophilic antibodies.For bivalent or dimeric target proteins, one may assess specificity by comparing assay signals with and without immunodepletion of the bivalent or dimeric target protein.
Reading an MSD Assay Protocol Plate
After adding the Read Buffer, avoid bubbles and analyze the plate with Sector PR or Sector Imager instrument. Following is an ideal parent figure MSD protocol for reading an MSD assay plate.
- Load the plate into a stacker or place it on a single-plate adapter
- Open the Discovery Workbench icon on the desktop
- In the upper left corner, click on the instrument icon
- Select the “Read from Barcode” menu from the plate type pull-down, in the case while reading a whole plate
- Check the “Read” icon to enter the number of plates
- Click the “Run” icon
- Select the desired export formats
- In the “Output Path” dialog field, select the location to save the results
- Click OK to begin the run
- Once the run is complete, data is saved automatically in the Plate Data History Database. The text version of the immunogenicity data will be saved in the designated folder
Some additional measures while reading assay plates are highlighted below.
- Bubble formation while adding a Read buffer to the wells can interfere with imaging the assay plate. Hence, use reverse pipetting techniques while adding the Read Buffer
- Plates can be assessed immediately after adding Read Buffer. However, the time between Read Buffer addition and plate imaging must be uniform to ensure consistency in ADA assays.
- Notably, regardless of the type of file export, immunogenicity data is always stored in the internal database. Hence, one may access and export the data from the Plate Data History database whenever needed
Thus, NorthEast Bioanalytical Laboratory is well-versed with the Meso Scale Discovery platform for MSD immunoassays. Besides the regular running of MSD assays and autoantibody biomarker discovery, we incorporate all additional measures to provide reliable, sensitive, and consistent results. Our expertise in immunogenicity testing in clinical trials ensures high-quality data, and our comprehensive immunogenicity bioanalytical services support various research needs. We are dedicated to delivering top-tier immunogenicity testing services, essential for advancing immunogenicity testing in clinical trials and ensuring accurate immunogenicity assessments.